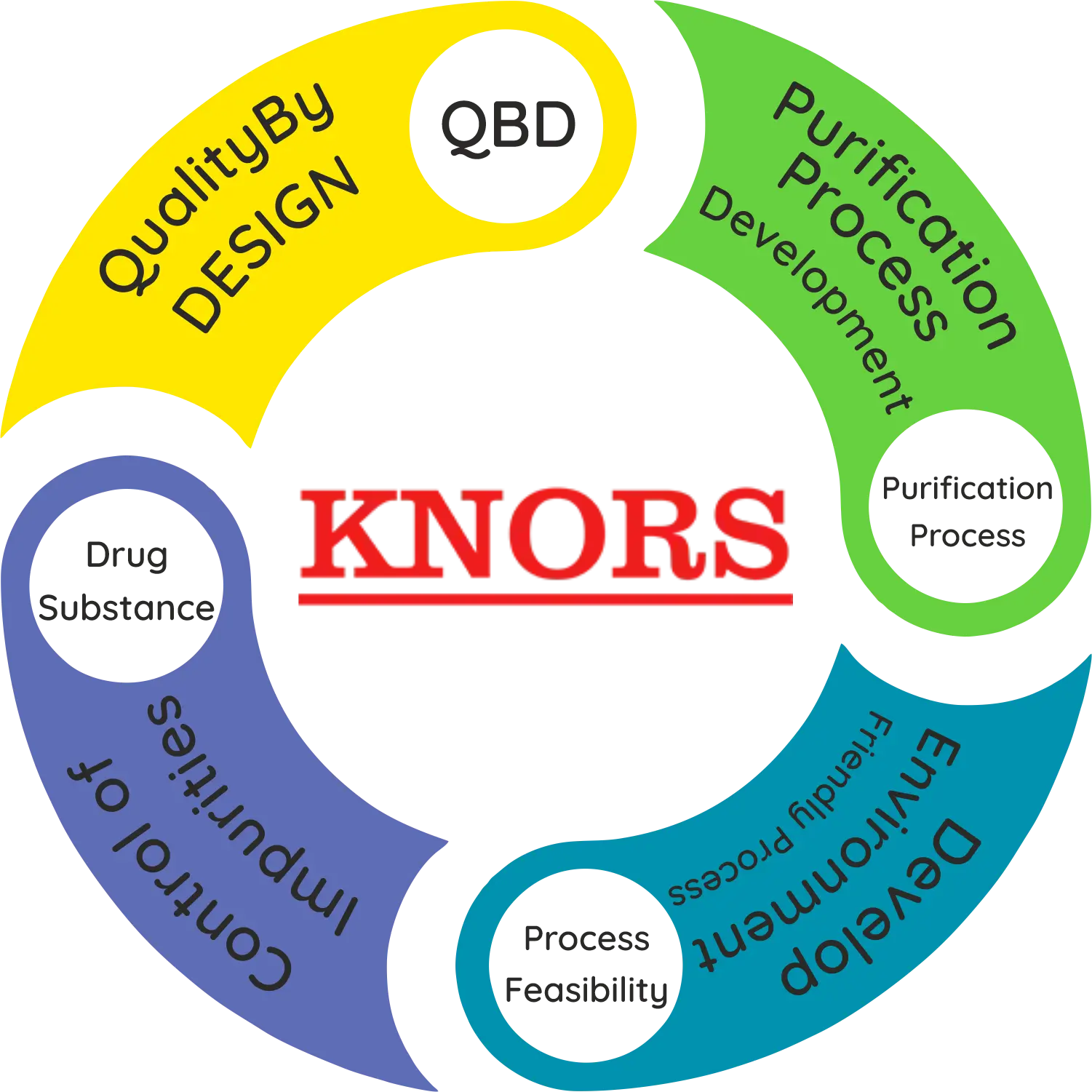
• Process development incorporating QbD (Quality by Design) approach
• Technology optimization by reducing the cost and enhancing the quality
• Chiral Synthesis and Isolation
• Purification Process Development
• Control of Impurities in the Drug Substance
• Supplier Development and its Qualification
• Process Feasibility Studies
• Substitution of toxic and hazardous chemicals to develop environment friendly process
• Technology optimization by reducing the cost and enhancing the quality
• Chiral Synthesis and Isolation
• Purification Process Development
• Control of Impurities in the Drug Substance
• Supplier Development and its Qualification
• Process Feasibility Studies
• Substitution of toxic and hazardous chemicals to develop environment friendly process